Abstract
Background: Currently, Peripheral Insertion Central Venous Catheters (PICCs) represents the device of choice for the treatment of hematologic patients because of lower risk of complications, easier management in outpatient population compared to Centrally Inserted Central Venous Catheters (CICCs) and effectiveness in performing high-risk therapy as hematopoietic stem cell transplantation.
Introduction: PICC insertion and use could be characterized by the occurrence of complications, whose most dangerous are the systemic and local infections and thromboembolic (TE) manifestations. The aim of this study is to evaluate the incidence of TE complications and the risk factors associated to their development.
Methods: Since the 2007, a PICC team, consisting of a hematology physician and three dedicated nurses has carried out a prospective study to evaluate complication rate and usefulness of PICC device in the hematology clinical practice. Inclusion criteria included all hematologic inpatient and outpatient who needed program of chemo-radiotherapy, support treatment and HSCT, regardless of white blood cells (WBC) and platelets (PLT) counts. All patients underwent a previous evaluation of arms vascular anatomy by ultrasonography. All implantation procedures were performed under ultrasound guide with radiographic control following insertion. The TE complications were diagnosed in clinical suspicion with venous color-doppler ultrasound and, if necessary, vascular CT scan. The therapy was performed according to local guidelines. The duration of PICC life is reported as 1000 days/PICC. The statistical analysis were performed with R software (R core team 2021).
Results: From March 2007 to June 2020, 1132 PICC implantation were performed in 983 hematologic patients (545 male and 438 females). Median age was 56 years, range 15-94. The most representative diseases were non-Hodgkin Lymphoma (NHL) (332 patients, 33,8%), Hodgkin Lymphoma (HL) (276 patients, 28%), Multiple Myeloma (MM) (127 patients, 12,9%), Acute Myeloid Leukemia (AML) (107 patients, 11%). Seven hundred and eighten PICCs (63.4%) were inserted in patients that underwent a previous therapy.
Nine hundred and ten PICCs (80,4%) were used for chemo-radiotherapy courses, 95 (8,4%) for support treatment, 111 (9,8%) for autologous hemopoietic stem cell transplantation (HSCT), 15 (1,3%) for allogenic HSCT, 1 for auto-allo HSCT (0.09%). PICC median life was 133 days (range 1-1257) for a total of 156485 days.
Forty-two PICCs were lost at follow up and for this reason the analysis about duration and complication was performed on 1090 devices. At the time of this analysis 25 PICCs (2,3%) are still in situ and in use, 754 (69,2%) were removed for end of therapy, 94 (8,6%) for accidental withdrawals, 130 (11,9%) for death, 1 (0,1%) for patient decision. Only 86 PICCs (7,9%) were removed because of catheter related complications: 4 (0,36%) for catheter rupture, 25 (2,3%) for malfunctioning/occlusion, 11 (1%) for malposition, 7 (0,64%) for local infection, 3 (0,3%) for confirmed PICC-related sepsis 36 (3,3%) for suspected PICC-related sepsis.
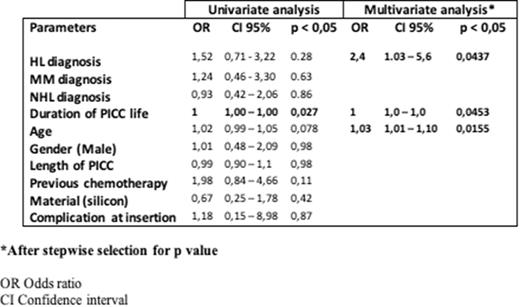
There were only 30 episodes (2,7%; 0,19/1000 days/PICC) of symptomatic PICC-related thrombotic complications, without need of removal. The TE complications were described in PICC inserted in patients affected by HL (11 PICCs, 36.3%), NHL (9, 30%), MM (5, 16,7%), AML (2, 6,7%), promyelocitic leukemia (2, 6,7%), non neoplastic anemia (1, 3,4%). The univariate analysis shown a strong association between the TE complications and the duration of PICC life (Tab. 1). The multivariate analysis confirm this data with a demonstration that a diagnosis of HL and the age at time of insertion could be considered as risk factors for the onset of TE disease (Tab.1).
Conclusions. These data shown that the rate of TE complications in our experience in adult hematologic patients is very low, no impacting on the safety of patients and usefulness of the devices. The advanced age of patients, duration of PICC life and HL diagnosis seem to be risk factor for the onset of TE manifestations PICC-related.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.